Girls Day at STScI on April 27, 2023: At one of the observation stations in the school lab, the young women were able to experience in their own experiment how astrophysicists gain knowledge with the help of spectroscopy.
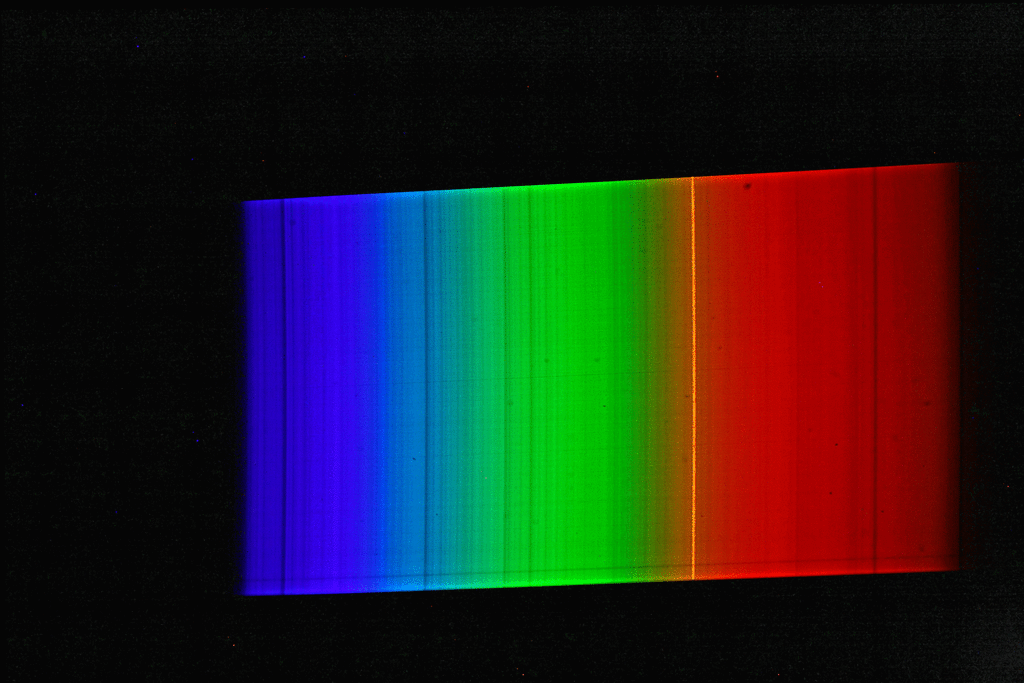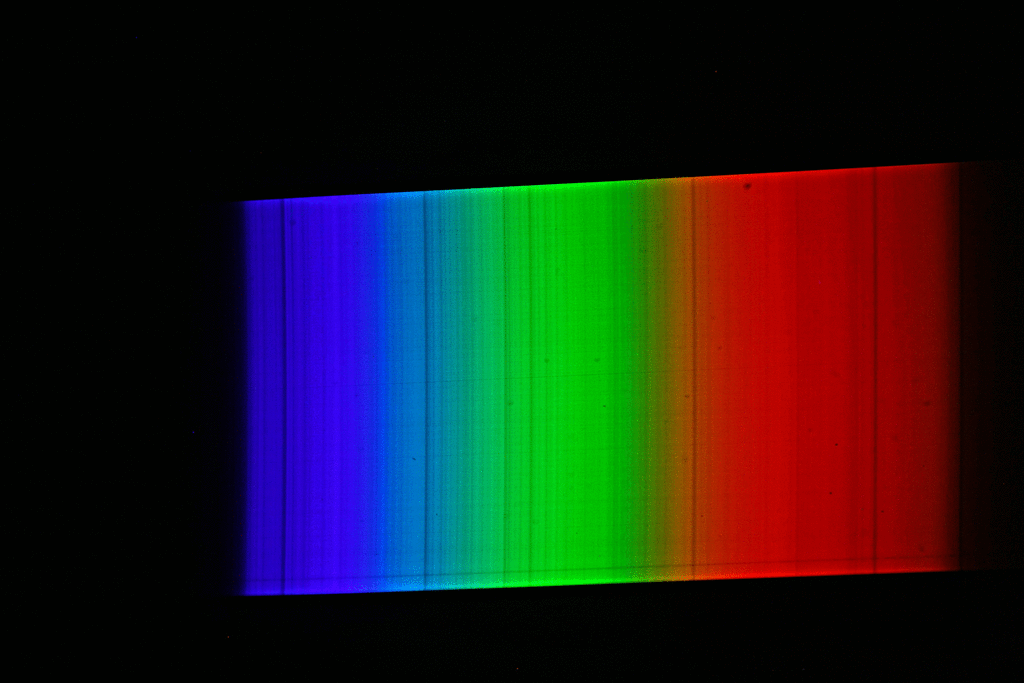
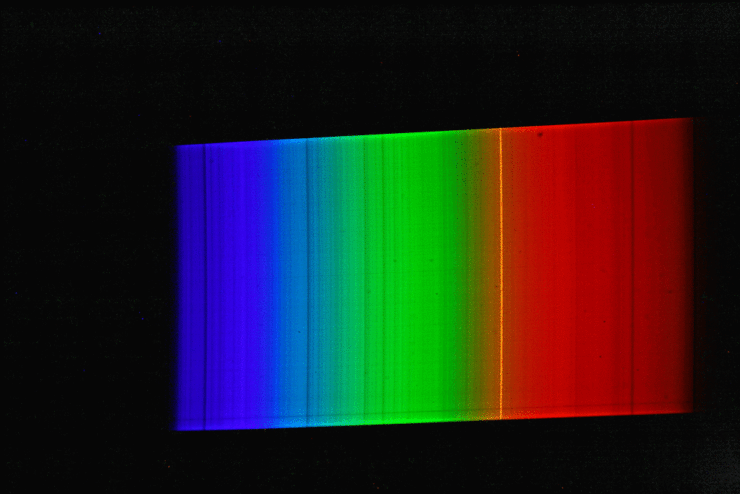
A spectrograph was first used to record a spectrum of sunlight. This results in a continuous spectrum with numerous “gaps” or dark lines, the so-called Fraunhofer lines. Here, certain narrow color ranges of the sunlight are “missing”, which are “withdrawn” from the sunlight reaching us mainly by absorption by gases in the sun’s photosphere, but also partly by absorption by gases in the earth’s atmosphere. Then the spectrum of luminous sodium atoms was recorded. The students scattered common salt crystals (sodium chloride) into the rather dark blue flame of a gas-powered blowtorch. This caused the gas flame of the lamp to glow with an intense yellow-orange light. The spectrum of this light shows only a single line (color).
If we now superimpose the solar spectrum and the sodium spectrum, we see that the yellow “sodium line” covers exactly one of the striking dark lines in the spectrum of the sun. The animated image illustrates this fact even more vividly.
The explanation of astrophysics: Somewhere on the path of sunlight from the surface of the sun to the surface of the earth there are numerous sodium atoms which absorb the yellow-orange light. We know that the Earth’s atmosphere contains virtually no sodium, and that the space between the Sun and Earth is largely empty. Consequently, the sodium atoms that “swallow” the yellow-orange sunlight must be in the direct vicinity of the sun (photosphere).
Such and similar spectroscopic methods have been and are being used to determine the composition of numerous objects in the universe.
Text: Peter Stinner





Add comment